Molar Absorptivity of Fe(II) Ferrozine Complex
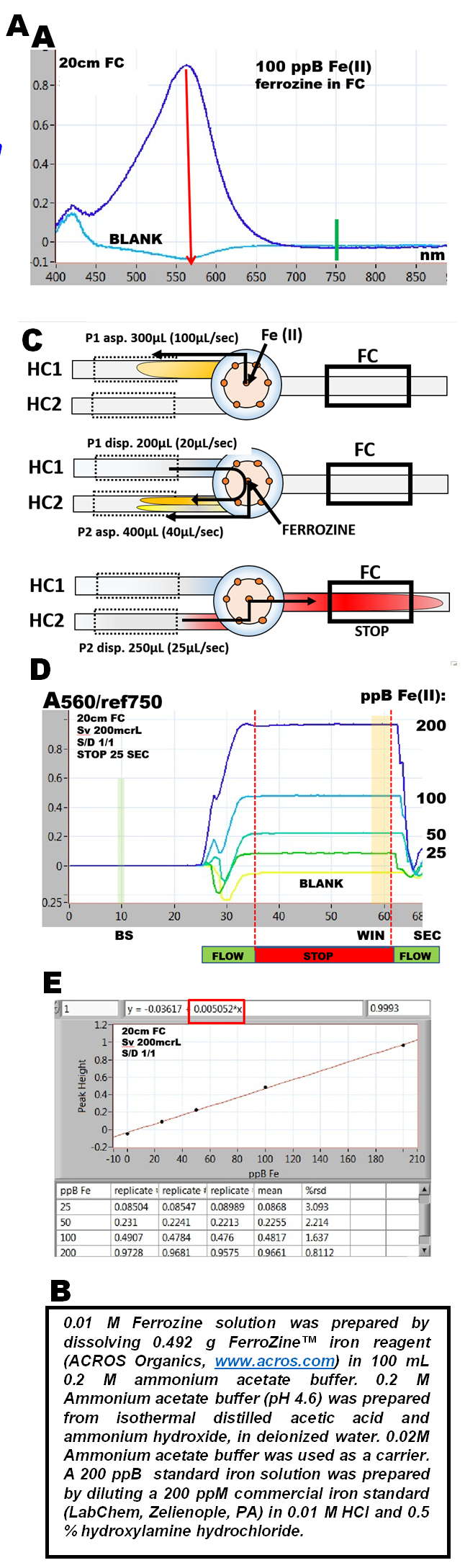
Ferrozine®, sodium salt of pyridyldiphenyltriazine sulfonic acid (m.w. 492.46) is related to the group of ferroin reagents, that all form water soluble chelates with iron (II). Red ferrozine-Fe (II) chelate adsorbs at 562 nm (A), being formed in acetate buffer at pH=4.5 in presence of hydroxylamine that serves as reducing agent (B). The flow programming for this determination is the same as for bromothymol blue, but in this case reaction between Fe(II) and ferrozine takes place in the flow system as these solutions are mixed at confluence point (C). By using the software protocol shown on the previous page, series of Fe(II) standards in the range of 0 to 200ppB Fe were analyzed and the absorbance values were recorded (D). Because the absorbance of response curves does not change during the stop flow period, it follows that chemical equilibrium has been reached. Therefore the absorbance values collected before the flow was resumed (WIN) represent a complete formation of Fe(II)ferrozine complex. The slope of the resulting calibration line (E) is
5.05 mAU/1ppB Fe(II)
measured in 20cm long light path, on a solution of Fe(II), diluted in ratio 1 + 1. This yields the molar absorptivity (Fe a.w.= 55.9) at 620nm:
ε = 2.83x10exp4
while the value listed in the literature (Cheng 1982) is:
ε = 2.86x10exp4
1.5.4.
L.Cheng, K. Ueno and T. Imamura: “Handbook of Organic Analytical Reagents” CRC Press. NY. 1982 p. 326.









